Answers to problems in "Electrode Potentials"
Question 8
ERRATUM: Kw = aH+ aOH- and not as printed in the book.
Both cells A and B are Harned Cells
| Pt | H2(g) | HCl(aq) | AgCl(s) | Ag(s) |
| RHE: | AgCl(s) + e- ⇌
Ag(s) + Cl-(aq)
|
| LHE: | H+(aq) + e- ⇌
½H2(g)
|
Cell Reaction:
| AgCl(s) + ½H2(g) ⇌ Ag(s) + H+(aq) + Cl-(aq) |
Nernst Equation: Ecell = E0cell - {RT/F}ln{aH+ aCl-},
for PH2 = 1 bar which approximates to 1 atm., and where E0cell = E0Ag|AgCl (by definition of E0H+|H2 = 0V).
Consider Cell A
aH+ = Kw/ aOH-
Hence, Ecell = E0Ag|AgCl
- {RT/F}ln{Kw aCl-/ aOH-}
Now, aX = fX[X], where fX is the activity coefficient of X (also given the lower case Greek symbol gamma) and, [Cl-] = [OH-] = c
Also, since c < 10-2M, the Debye-Hückel Limiting Law (DHL) applies.
Since lgfi = -Azi2I½, and I, zi2 and A are all the same for both Cl- and OH-, fCl- = fOH- and so,
| Ecell = E0Ag|AgCl - {RT/F}lnKw | (1) |
From the data given, we notice that Ecell A is not a function of concentration (as equation (1) predicts), and therefore,
| 1.0493 = E0Ag|AgCl - {RT/F}lnKw | (2) |
We next need a value for E0Ag|AgCl which (if we are to find Kwmust therefore be obtained from cell B.
Now we consider Cell B:
Ecell = E0Ag|AgCl
- {RT/F}ln{aH+ aCl-}
aH+ = f+c and aCl- = f-(2c)
Using the DHL, we see that f+ = f-
= f , since zi2, A(= 0.509 in water at 298K)
and I are the same for both H+ and Cl-, as
they co-exist in a solution of ionic strength I.
Therefore, E = E0Ag|AgCl - {RT/F}ln{f2 2c2}
E + {RT/F}ln{2c2} = E0Ag|AgCl - {4.606RT/F}lg(f)
E + {RT/F}ln{2c2} = E0Ag|AgCl - 4.606(0.509)RT(3/2)½ c½/ F
Hence, a plot of E + {RT/F}ln{2c2} (on the ordinate axis) against c½ (on the abscissa) will have an ordinate intercept equal to the Standard Electrode Potential for Ag|AgCl.
| E/V | c/mol dm-3 | c½/ mol½ dm-3/2 | E + {RT/F}ln{2c2}/V |
|
0.578 |
0.001 | 0.0316 | 0.2411 |
|
04969 |
0.005 | 0.0707 | 0.2426 |
|
0.4624 |
0.010 | 0.1000 | 0.2437 |
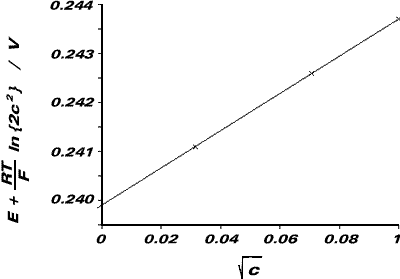
From the graph, ordinate intercept = E0Ag|AgCl = +0.2395V
Thus, from (2), Kw = 2.00 x 10-14 mol2 dm-6.
Of the course the true answer for Kw is only one half as big; students 'refining' their results beware!



