Research
Gas Sensing
Gas sensing in a variety of media constitutes a significant proportion of work carried out by the Compton group. The gases studied within the group range from the benign, such as oxygen and hydrogen, to the much more toxic gases, such as ammonia, hydrogen sulphide, sulphur dioxide, nitrogen dioxide and chlorine. The detection of hydrogen gas is of great interest due to its major importance in fuel cells and other applications. The detection of more toxic gases is of great importance due to their detrimental effects on human health and their prevalence in various industries. Hydrogen sulphide, sulphur dioxide and nitrogen dioxide are all pollutants that have been linked to respiratory problems as well as contributing to increased mortality. Hydrogen sulphide is a well-known hazardous pollutant which is found naturally in coal pits and oil and gas wells, while the emission of sulphur dioxide and nitrogen dioxide is a major contributor to acid rain in addition to other health risks.
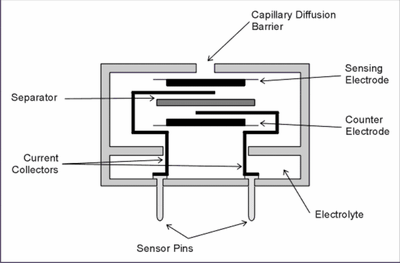
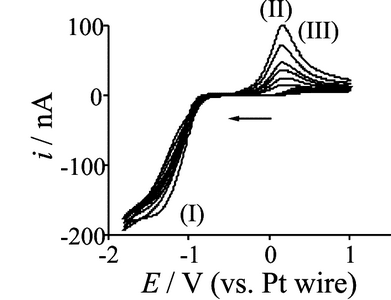
One of the most common designs employed for commercial gas sensors is based on a cell where the analyte gas diffuses through a gas-permeable membrane into an electrolyte solution where it is detected potentiometrically or amperometrically. Such gas sensors tend to employ conventional solvents such as a sulphuric acid/water mixture; however, a gas sensor lifetime is determined by how quickly the electrolyte dried out, and changeable temperatures and pressures can render conventional solvents to be unstable and volatile. Room temperature ionic liquids (RTILs) have recently been employed as a supporting electrolyte for gas sensors as they are highly stable and non-volatile, and can survive drastic temperature and pressure changes. Therefore, sensors employing RTILs can be used in extreme climates and still function. While the high viscosity of RTILs results in slower response times, their use eliminates the need for supporting electrolyte. The electrochemistry of all the gases mentioned above has been observed in RTILs utilising microelectrodes as the working electrode. The use of microelectrodes allows us to attain steady-state currents and relatively faster response times, as well as reducing the background current, signal-to-noise ratio and allowing for a smaller sensor. This has allowed better understanding of redox properties of each species, and efforts are underway to fabricate a commercial gas sensor to overtake those that currently employ more volatile solvents.
Due to the resilience of RTILs to changing physical conditions, we will take gas sensors where no gas sensors have gone before.