Research
Electroanalysis
The use of voltammetric techniques in analytical chemistry is a key application of electrochemistry. The methods are highly appealing to the analytical chemist owing to their high sensitivity, low cost, simplicity of instrumentation and ease of implementation.
Numerous analytes are determined using electroanalytical methods, ranging from toxic heavy metals (e.g. cadmium, lead), to simple organic molecules. Some commonly known widespread applications of electroanalysis are in the determination of blood glucose levels to monitor diabetes, and ethanol sensors to determine blood alcohol levels in breathalyzers.
More recently, the group reported the highly popular electrochemical chilli sensor for the determination of capsaicin-the molecule responsible for making chillies hot! The carbon nanotubes modified electrode proved highly sensitive to the amount of capsaicin, and gave very agreeable results with respect to the current Scoville method, and a range of very hot sauces!
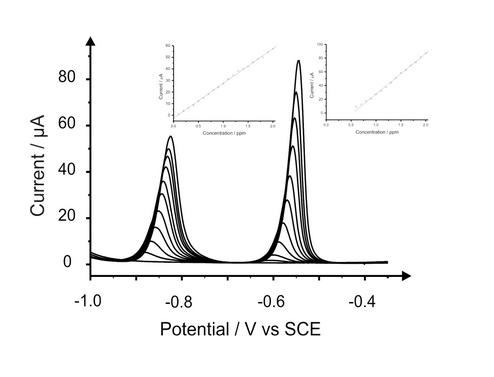
The group is continually developing electroanalytical sensors to improve on current limits of detection, or investigate the analysis of completely new analytes. The work involves a range of voltammetric methods, including cathodic and anodic stripping voltammetry, adsorptive stripping voltammetry and chronoamperometry. With a vast range of analytical techniques available, the analytical prowess of numerous modified carbon based and metal electrodes can be fully explored.
Much work in the group in recent years has utilized the relatively new electrode material, boron doped diamond, publishing a significant amount of the literature regarding electroanalysis at this highly diverse and unexplored electrode. Boron doped diamond (BDD) possess all the electrochemically alluring properties of diamond, but is highly conductive due to the boron doping, therefore making the almost atomically flat, chemically inert and robust substance an ideal electrode material. Recent publications regarding BDD have explored metal nanoparticle modification of the electrode surface to enhance analysis towards key analytes. Another key electrode material featuring heavily in the group's recent publication history is carbon nanotubes.
Ionic liquids also offer great applicablilty to electroanalysis, with much work occurring within the group regarding the determination of gases, such as toxic hydrogen sulfide. Such research is highly innovative, as the determination of gases is notoriously difficult electroanalytically in conventional electrolytes. The importance of electroanalysis is evident throughout the group, as theory, thermodynamics, and novel materials come together to utilize an essential application of electrochemistry.