Research
Electron Spin Resonance Spectroscopy
EPR spectroscopy was first applied to electrochemistry, albeit at low temperature, in 1958 by Ingram et al., who demonstrated the formation of radical ion species from the potentiostatic electrolysis of various aromatic compounds in N,N-dimethylformamide (DMF). The ability of the in situ electrochemical EPR technique to provide information on kinetics and mechanisms of electrode processes is the major reason for its adoption by electrochemists although its major limitation is that it requires the presence of a paramagnetic reaction intermediate.
In Situ Co-axial Flow Cell: Cell Design
Waller and Compton effectively mimicked the Allendoerfer co-axial design, whilst simultaneously maintaining the mathematically well-defined laminar flow of the channel flow cell. This improved cell for electrochemical EPR allowed an improvement in the channel cell regarding lifetimes of radicals amenable to study, whilst retaining the hydrodynamic flow that is essential for the investigation of electrode reaction mechanisms.
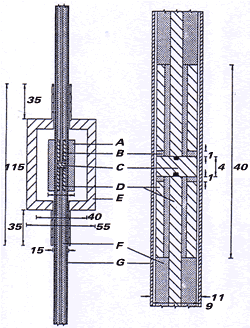
The cell itself (see below) comprises a TE011 cylindrical cavity which is converted into a co-axial cavity by the addition of a smooth, polished copper rod that is positioned centrally within the cavity. The rod itself (diameter 9 mm) is located within a precision-bore silica tube, so that there is an annulus (of size 2h) around the rod, constrained to be of uniform thickness by a fine nylon thread running the length of the cavity. Typically 2h ~ 100 mm, but this is adjustable by polishing the rod. The central 4 mm of the copper rod is insulated from the rest of the rod, as this part is the working electrode. To ensure a large negative potential window, this central part of the rod was plated with mercury. As in the case of the channel cell, this cell was connected to a reservoir-fed gravity flow system, with a platinum gauze counter electrode located outside the cavity and sufficiently away downstream so as to preclude counter-electrode products from diffusing into the cavity, and an upstream reference electrode. Electrolyte solutions flow through the annular gap between the copper rod and the silica tube. Flow rates within the ranges 10-4 to 10-1 cm3s-1 were obtained in this manner. Since the flow pathway is narrow and resistive, the potentiostat was modified so as to provide high voltages to drive the current through the channel. Electrolyte resistance in the narrow gap through which the solution flows may lead to a non-uniform potential difference (vide supra), affecting the voltammetry only in poorly-conducting non-aqueous solutions using low currents.
Like the Allendoerfer co-axial cells, it was found that the resonant frequency of the cavity shifted above that of the empty cavity and outside the klystron tuning range due to the reduced effective cavity size produced by the addition of the copper rod. As above, this problem was eliminated by partially filling the cavity with a material of appropriate relative permitivity (such as a PTFE shielding around the silica tubing), shifting the resonant frequency back to typically 9.4 GHz.
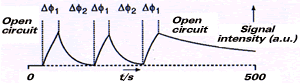
The sensitivity of this modified cavity, as observed with a small crystal of DPPH, was found to follow a sin2 function along the length of the cavity and at a fixed distance from the copper rod, as anticipated theoretically. However, the EPR sensitivity was observed to vary in a cos2 manner around the copper rod at a fixed distance from the cavity. Since the cylindrical cavity sets up cylindrically symmetric standing microwave field patterns, this behaviour can only be rationalised by a perturbation of the component of the magnetic field that is modulated at 100 kHz, caused by eddy currents induced in the copper rod by the 100 kHz component. Lenz's Law requires the induced magnetic field to oppose the applied field, and this effect is maximally observed when the applied field is perpendicular to the copper surface, and zero when it is parallel to the copper surface. This cos2 variation in sensitivity has also been observed in the Allendoerfer co-axial cell. This effect inevitably means that paramagnetic species will experience different amplitudes of the modulated field depending upon their location within the cell. However, it was shown that this will reduce the EPR signal by only a factor of one half from that which would be otherwise seen.