Research
Fast Scan Voltammetry
In recent years there has been a dramatic shrinkage of the voltammetric timescale brought about by the introduction of microelectrodes. These microelectrodes possess at least one dimension of a size no more than the micron scale, resulting in greater rates of diffusion to the electrode surface. As a result of this microelectrodes are able to study ultrafast heterogeneous kinetics and homogeneous events coupled to electrochemistry that were previously masked by transport control and therefore outside the scope of measurement by voltammetric means.
Study of Heterogeneous Kinetics
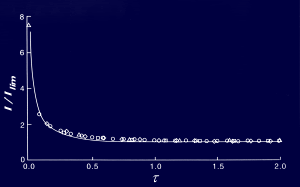
Experiments were conducted on solutions of p-benzoquinone (J. Phys Chem, 1995, 99, 14817) (BQ) in 0.1M TBAP/acetonitrile using the fast flow channel flow cell. BQ was found to give a reduction wave with a half wave potential of -0.555V (vs Ag reference electrode). The flow variation of the transport limited current was found to obey the Levich equation with D = 2.57 x 10-5 cm2s-1. Full current voltage curves were then recorded for different flow rates and mass transport. Tafel analysis was conducted for each voltammogram. The mass transport Tafel plot for a flow rate of 2.20 cm3s-1 is shown below. The standard rate constant was found to be ks = 0.30 ± 0.04 cms-1. This is in excellent agreement with an independent value of 0.24 which was derived from fast scan microdisk cyclic voltammetry (Anal. Chem., 1984, 56,524). It has been demonstrated that the fast flow channel flow cell is capable of measuring rapid standard electrochemical rate constants from steady-state experiments. The power and versatility of this new technique in investigating fast electrochemical processes is apparent.
Study of Homogeneous Kinetics
We next turn the application of the high speed channel electrode to the study of fast kinetic processes coupled with electron transfer processes. The reaction considered is that of bromobenzophenone (pBrBP) which undergoes the ECE mechanism.
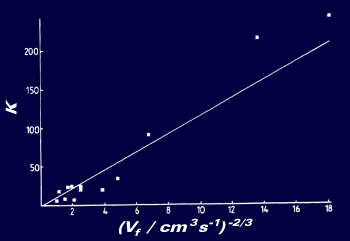
A conventional channel electrode was used to record reduction waves over a range of flow rates in order to find the diffusion coefficient and reduction potential of pBrBP: D = (7.4 ± 0.5) x 10-5 cm2s-1 and E0 = -1.530 ± 0.02V (vs Pt). Voltammograms were then recorded for the reduction of pBrBP in 0.1M TBAP/DMF solution at a 5μm Pt microband using the fast flow channel flow cell. ECE analysis was performed by plotting a graph of the variation of Neff., the effective number of electrons transferred in the ECE reaction, with flow rate. Neff varied from almost two electrons at slow flow rates to nearly one-electron at high flow rates. Neff is a unique function of a dimensionless rate constant KECE.
The use of the ECE working curve enables Neff. to be turned in to KECE values. These are then plotted against flow rate-2/3 to obtain a graph with a gradient from which the first order rate constant can be inferred. The rate constant, k, was found to be 76,500 ± 20,000 s-1. In conclusion, the use of microband electrodes in conjunction with the fast flow channel flow cell allows rate constants of the order of 105 s-1 to be measured. These results enter a new time domain in steady-state electrochemical measurements.