Research
Laser Activated and Photo-voltammetry
When the surface of an electrode is illuminated with a high power laser (0.1 Wcm-2 to 0.8 Wcm-2), several phenomena take place, most of them derived from the heating of the electrode surface due to the adsorption of the laser energy by the electrode material. The sudden jump in the temperature of the electrode surface after the laser pulse deeply alters the properties of the electrochemical interphase, causing desorption of adsorbed species, convection of solution, renewal of the diffusion layer, etc. At even higher laser powers the energy becomes enough to cause the melting or even the vaporization of the outer layers of atoms on the electrode surface, followed by optical emission, sound and heat generation and generation of fine metal particles and gas bubbles.
The term "activation" is used to distinguish the depassivation and current enhancement effects while maintaining an essentially undamaged electrode from the drastic current fluctuations and major electrode damage associated with the "ablation" obtained with the higher laser intensities.
Laser illumination can used to activate or refresh an electrode surface when the presence of passivating species would tend to deactivate it. The renewal of the diffusion layer in each laser pulse causes an enhancement of the mass transport. Combination of both effects opens a wide range of possibilities both for physicochemical studies (kinetics, mechanisms of reactions involving poisoning intermediates, etc) and for its application to electroanalysis. These effect should be distinguished from the direct chemical effects and a contrast can be drawn with situations where either solution phase species absorb laser light to form electronically excited species which then lead to electrochemical processes or where irradiation of a semiconducting electrode is used to generate charge carriers.
Electrode Depassivation and Analytical Applications
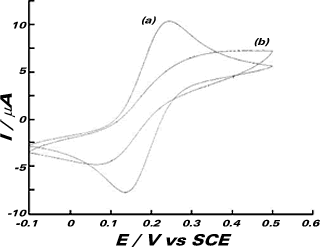
Having successfully used laser activation to measure half-wave potentials and limiting currents, attention was then turned to the application of the LAV for analytical purposes in passivating media. In order to check this application the reversible oxidation of ferrocyanide in the presence of surface passivating species that are electroinactive was chosen as a model system. The blood proteins fibrinogen and bovine albumin (BA) were used for this purpose. An aqueous solution of 10 mm K4[FeCN6] in 0.1 M PBS along with 10 gL-1 BA and 1 gL-1 fibrinogen was adjusted to pH 6.81. Dissolution of the mixture was aided by 20 minutes of mechanical stirring to form a pale yellow, viscous solution. Cyclic voltammetry at 50 mVs-1 was performed on the solution to investigate the effects of the added proteins. A typical scan in the presence of protein is shown, along with a comparison scan in the absence of bovine albumin and fibrinogen.
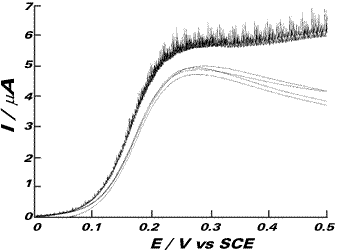
The waveshape and peak currents rapidly deteriorate with successive scans (the scan shown is the fifth in a series of ten) and we attribute this to the surface adsorption of electro-inactive protein molecules which gradually block the electron transfer from the ferrocyanide ion and reduce its reversibility. To investigate the effects of laser activation on this passivating layer, linear sweep voltammetry at 20 mVs-1 was performed both in the dark and subject to 500 mW cm-2 laser irradiation. The figure on the right shows six consecutive scans, the first and last of which are subject to illumination by the laser and retrace the same path after the four diminishing dark scans, proving in this way the beneficial effects of the laser irradiation to keep clean electrode surfaces during electroanalytical applications.