Research
Liquid|Liquid Electrochemistry
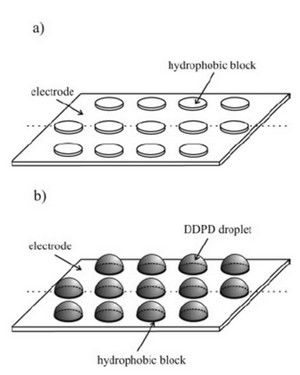
Liquid|liquid interfaces are present in a large number of systems varying from simple emulsions to complicated biological assemblies, so their study is of vital importance in a wide range of disciplines. In recent years we have developed an experimental technique that allows us to electrochemically probe reactions which occur across the oil|water interface. In particular, we are able to study reactions of both solutes dissolved in oil as well as electroactive oils directly. A newly designed partially-blocked electrode (PBE) has been shown to provide an excellent means to study biphasic systems. The PBE consists of a gold electrode covered with a regular array of non-conducting hydrophobic polymer blocks. Electroactive oils such as butylferrocene and long-chain tetraalkylphenylenediamines preferentially form hemispherical microdroplets on the polymer blocks which persist when immersed in aqueous electrolyte solution. Oxidation then results in the transport of ions across the oil | water interface: either anions from aqueous phase into the oil droplet, or organic cations from the oil to aqueous phases. This can be detected voltammetrically and provide by comparing with simulations we can determine important features of the mass-transport (i.e. diffusion, migration).
By dissolving a solute in an inert oil, we can study biphasic reactions to investigate what role, if any, does the liquid|liquid interface play in biphasic reactions? Does the liquid|liquid interface enhance, slow down or have no affect on the rates of reactions? Can the liquid | liquid interface somehow encourage certain side reactions to be dominant? Our work provides insight into this relatively unexplored field, e.g. we have discovered a solvent-polar effect that dramatically slowed down the reaction of Co(I)L (reduced vitamin B12) with acyclic vicinal dibromides but had little effect on the corresponding reaction with cyclic dibromides.
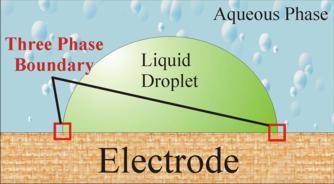
Electrochemistry at the Three Phase Boundary
Modification of electrodes with electroactive liquid microdroplets provides a simple methodology with which to elucidate the mechanism of electrochemical oxidation or reduction in the biphasic environment. An important feature of this work is the fact that, in contrast to the traditional electrochemical approach where electrolyte was added to both liquid phases, the redox liquids of interest are unsupported and hence electrically insulating, so unless electrolyte ions partition into the microdroplets, electron transfer is confined initially to the annular region where the oil phase, the electrode and the aqueous electrolyte phase meet. This electrode / aqueous solution / organic droplet junction is termed the three phase boundary.
A wide variety of systems have been investigated utilizing the microdroplet methodology (including Phenylenediamines, Nitrophenyl Nonyl Ether, Vitamin K1 and Vitamin E). In addition, a review by Wadhawan and co-workers has been published which gives an overview of the technique.