Research
Photoelectrochemistry
A metallic electrode is irradiated with laser light to induce photochemical reactions of electrolytic intermediates such as anion or cation radicals so as to generate new chemistry or unusual reaction intermediates. In this way, for example, we can bring about the efficient dehalogenation of aromatic molecules - an observation which may provide a method for the clean up of toxic wastes such as PCBs. Additionally we are looking at the photoelectrochemistry of a wide range of organometallic species. Spectroscopic measurements, notably ESR and fluorescence, made simultaneously with electrolysis assist our mechanistic insights.
Photodisproportionation: Photoelectrochemical Reduction of Fluorescein
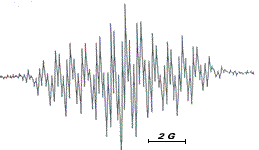
The electro-reduction of the green dye fluorescein, F, in basic aqueous solution at mercury electrodes leads to the formation of the semi-fluorescein radical, S1, which has the characteristic EPR spectrum shown in figure on the far left, in this case for reduction at a mercury-plated copper channel electrode located at the centre of a TE102 EPR spectrometer cavity.
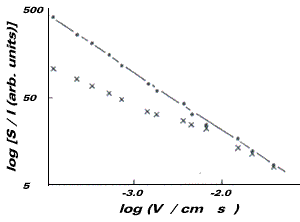
Such measurements permit the assessment of radical stability by means of monitoring the EPR signal intensity as a function of electrolyte flow rate through the channel electrode cell. The middle figure shows the results for the case of fluorescein which are seen to be in excellent agreement with equation 17, on which basis it may be inferred that the S1 radical is kinetically stable on the channel flow cell timescale and must therefore have a lifetime of at least a few seconds. However irradiation of the electrode with light of wavelength 390nm caused large photocurrents to flow and depletion of the EPR signal as shown in the middle figure. The formation of leuco-fluorescein, L, was deduced and the behaviour in the presence of light attributed to a disproportionation reaction.
The photocurrent arises from the regeneration of F which is then free to undergo further reduction at the electrode. The precise mechanism of the transformation was inferred by means of photocurrent and EPR signal intensity data measured as a function of solution flow rate. In each case the conclusion was that the photo-DISP2 mechanism was operative.
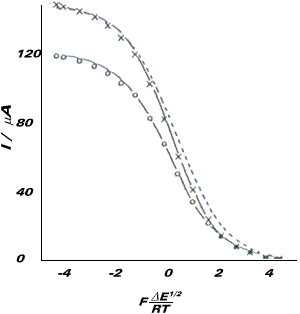
The disproportionation step was demonstrated to be rate determining and the proton transfer step to take place after the slow step in the reaction. As a final and independent verification of the deduced kinetics and mechanism the channel electrode modelling techniques were employed to compute the current-voltage waveshapes for the photo-DISP2 process. A simulated voltammogram is shown in the right hand figure (drawn as xxx) and relates to a flow rate of 1.5 x 10-3 cm3 s-1. Also shown in the figure is the voltammogram for the dark reaction - a simple electrochemically reversible one electron reduction (drawn as ooo) and also the latter voltammogram scaled to give the same mass transport limited current. Note that the effect of the second order reaction following the reversible electron transfer is to induce an asymmetry in the waves. This asymmetry becomes more pronounced as the flow rate is decreased and, in addition, the halfwave potential shifts anodically.