Research
Room Temperature Ionic Liquids
Room temperature ionic liquids (RTILs) are low temperature molten salts which are composed entirely of ions. The salts are characterized by low interaction between bulk unsymmetrical cations and weakly coordinated inorganic anions. This results in low tendency to crystallize at room temperature. They have numbers of properties including wide electrochemical windows, high electrical conductivity, high chemical and thermal stability and favorable solvating properties.
There are numbers of advantages using ionic liquids in electrochemical systems. The wide electrochemical windows which are dictated by the oxidation of the anion and the reduction of the cation enable us to perform electrochemical measurements at extreme potentials which can not be done using traditional solvents. Since RTILs have good intrinsic conductivity, they can be used as supporting electrolyte and that would minimize waste. RTILs are also called green solvents in this respect. The relatively high viscosity of RTILs which slows down the mass transport properties of electroactive species contributes to the change of voltammetric response of the species under study.
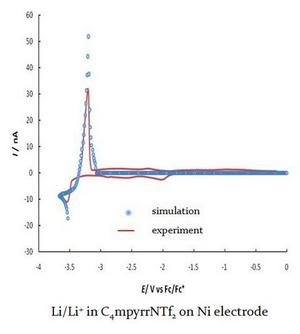
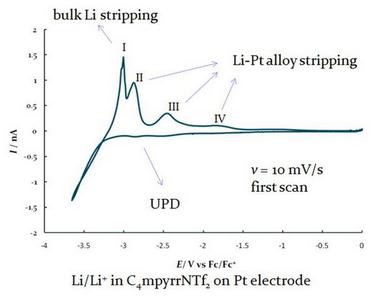
We currently employ these properties to be applied in electrochemical systems for gas sensing, examining mechanistic pathways of the electrochemistry of species (nitrobenzene, 4-nitrophenol, nitrate, ammonia, benzoic acid, etc) and studying the deposition/dissolution of some active metals (lithium, sodium).